- Home
- Technical Products
Enterprise Cloud IT Solutions
Test Measurement
Industrial Measurement
- Solutions
Enterprise Cloud IT Solutions
Test Measurement
- Latest Articles
- About Us
 EN
EN
Cold Chain Logistics Temperature Monitoring Solutions
Ensure compliance and product stability in the pharmaceutical supply chain from the manufacturing end to the patient end.

Global Pharmaceutical, Healthcare, Life Sciences and Biologics Logistics Markets
The production lines for advanced therapies (cellular and genetic) and biologics are huge. Due to their highly personalized nature, most of these medical treatments require high-quality, ultra-low-temperature storage and transportation. Any mistake in the refrigeration or transportation of these therapies could be life-threatening for the patient. The global growth potential of this new specialty healthcare product is exponential. It is critical to adopt new technologies to control the temperature-sensitive transport and storage of these personalized medications. The COVID-19 pandemic has accelerated the development of a new global infrastructure for ultra-low temperature management. Warehousing facilities, storage and cold storage have increased around the world. Due to the highly sensitive nature of these therapies, especially during transportation, highly reliable temperature monitoring and real-time solutions are required to provide end-to-end visualization of the supply chain for transportation.
Cold Chain Logistics and Temperature Monitoring Applications
All pharmaceutical products, including cellular and gene therapy (CGT), have stabilization budgets. Therefore, under GDP regulations, these products are considered temperature sensitive and need to be monitored. The diagram below illustrates several examples of pharmaceutical logistics, many of which involve warehouse personnel responsible for temperature control. Temperature must be monitored at every point in the supply chain. This may include integration into packaging, transportation vehicles and even storage facilities for drugs, CGT and vaccines.
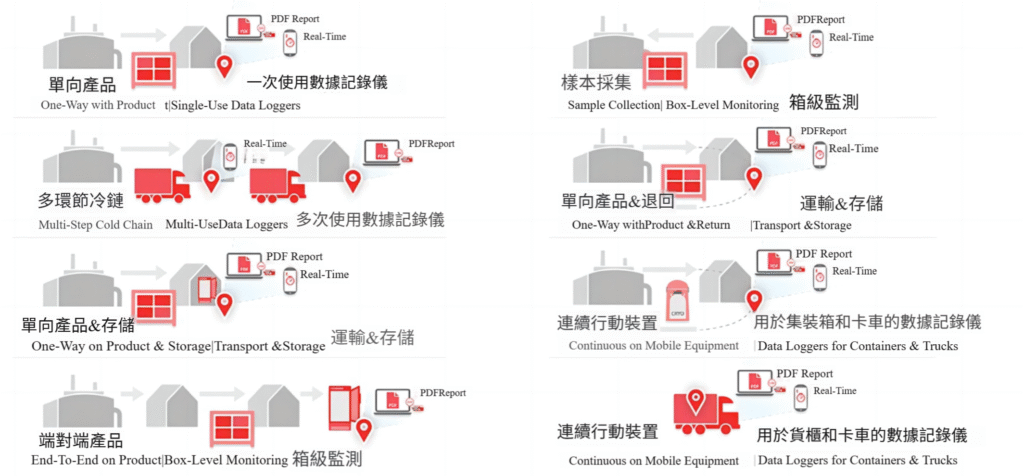
What is Cold Chain Distribution in the Supply Chain?

There are many complex temperature monitoring scenarios involved when considering cold chain distribution in the supply chain. Several temperature monitoring scenarios for global distribution may include the following data loggers or monitoring devices:
- One-way products (single use)
- Multi-storey cold chain warehouse (multiple use)
- One-way product and cold storage (single use)
- End-to-End Products (Box Level Temperature Monitoring Indicators)
- Sample collection (box-level temperature monitoring indicator)
- One-way products and returns (disposable or electronic temperature indicators)
- Continuous monitoring of mobile devices (real-time monitoring on containers and/or transportation vehicles)
What are the supply chain qualification requirements?

For cold chain products and equipment used in transportation, anything used to store or transport products in a GDP or GMP environment must be qualified. This applies to containers, reefer containers, airplanes, trucks and truck fleets, and even trade lanes. Qualification is the process of proving that a piece of equipment, a room, a transportation lane or even an entire network is fit for purpose. Confirmation of an entire network is much more complex and requires extensive analysis and documentation of the process than confirmation of equipment such as cryogenic shippers or freezers and rooms.
Hongke ELPRO Cold Chain Monitoring Solution for Transportation Products

For Cold Chain Logistics
PDF Data Logger and Indicator
The PDF reports generated by HONGKE ELPRO LIBERO devices are the most flexible and cost-effective way to monitor drug shipments.
- Washable PDF Data Logger
- Multi-purpose PDF/Bluetooth Data Logger
- Electronic Indicator
- GAMP5 compliant
- Compliance with FDA CFR 21 Part 11
- Alarm Display & PDF
- Manual readout
- PDF report
- IATA compliant batteries
- Free calibration certificate
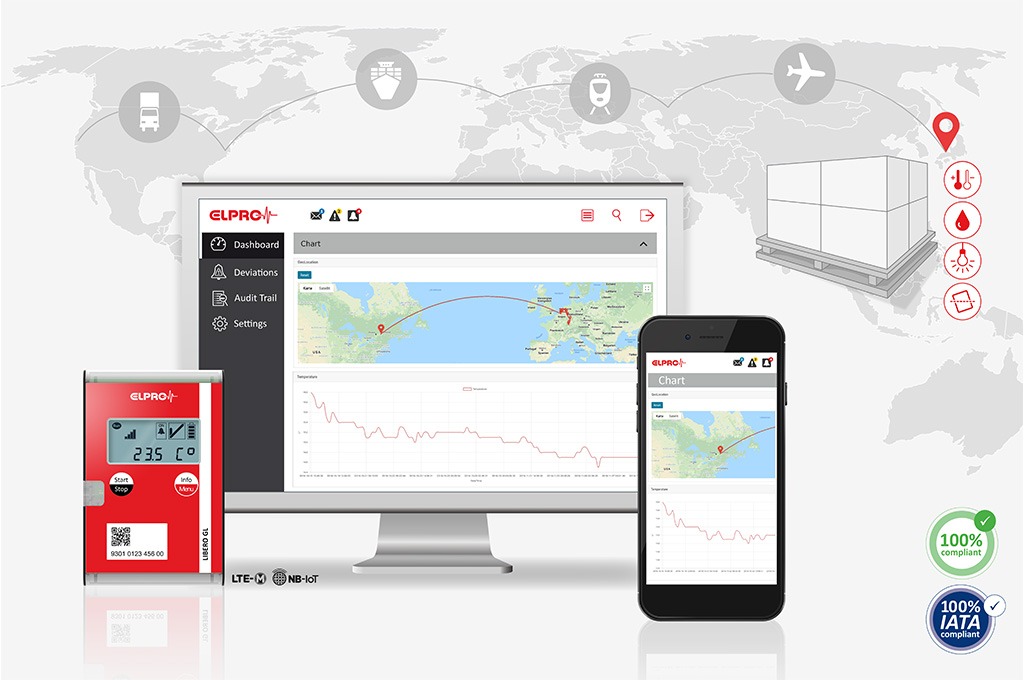
Transportation and Storage
Real-time temperature monitoring
Avantech ELPRO LIBERO Gx devices track temperature-controlled goods in real time over a public mobile IoT network. They can provide you with optimal management of your cold supply chain, including trucks, trains, ships or airplanes, as well as monitoring directly in product packages or low-temperature containers.
- Disposable Real-time Data Logger
- Multiple use of real-time data logger
- Data Logger for Containers and Trucks
- GAMP5 compliant
- Compliance with FDA CFR 21 Part 11
- 24/7 alerts via email and text message
- Auto-Readout
- Automatic Archiving and Reporting
- IATA compliant batteries
- Free calibration certificate, recalibration option
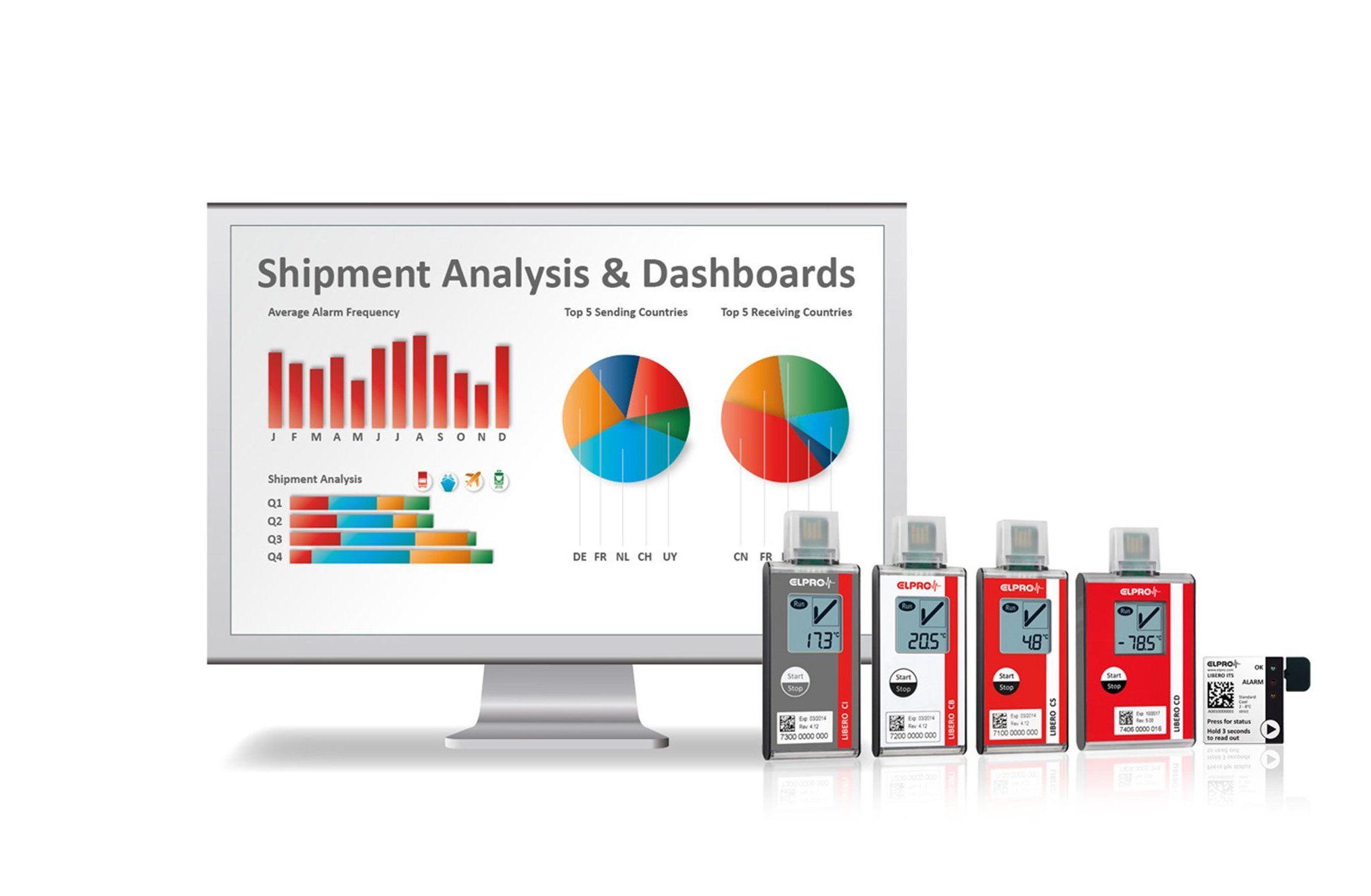
Cold Chain Database Achieves Excellence
Medical Flow Temperature Management
With the liberoMANAGER solution, excellent pharmaceutical logistics and supply chain management are at your fingertips. Track shipments in real time, manage temperature data and assess deviations, automate notification processes, and centralize device management pages.
- No-wash data logger
- Multiple use data logger
- Electronic Indicator
- GAMP5 compliant
- Compliance with FDA CFR 21 Part 11
- Email Alerts
- Manual/Automatic Reading
- Automatic Archiving and Reporting
- IATA compliant batteries
- Free calibration certificate

