- Home
- Technical Products
Enterprise Cloud IT Solutions
Test Measurement
Industrial Measurement
- Solutions
Enterprise Cloud IT Solutions
Test Measurement
- Latest Articles
- About Us
 EN
EN
TrackSense Pro-Rugged Flexible Temperature Sensor

Rugged Flexible Temperature Sensors
The rugged flexible temperature sensor is extremely robust and has a temperature range of -50 to +140 °C. It can be used in a variety of applications in the food and pharmaceutical industries and is particularly suitable for harsh conditions and in hard-to-reach areas of the environment.
The TrackSense Pro DataCORDER series is equipped with an interchangeable sensor option that unlocks multiple measurement parameters:
✓ Temperature
✓ Pressure
✓ Conductivity
✓ Relative Humidity
✓ Vacuum
✓ Vapor penetration
The DataCORDER can be easily activated and read by multiple readout stations and combined with the Sky Module via wireless communication to obtain real-time data.
- Technical Parameters
| Sensors with this logger configuration: | Rugged Flexible Temperature Sensors | Recorders with this sensor configuration: | LAN 3G |
|---|---|---|---|
| Temperature measurement range: | -50 to +140°C | Working temperature: | -50 to +140°C |
| Measurement Principle: | Resistance | Operational pressure: | 0.001 mBar to 10 Bar ABS |
| Sensor element: | Pt1000 | Surface material: | 316 Liter Stainless Steel |
| Cable Diameter: | 4 mm | Diameter of the logger: | 25mm |
| Cable length: | 500/1000 mm | Recorder length: | 44mm |
| Sensor Diameter: | 2.5 mm | With battery weight: | 48g |
| Sensor length: | 30 mm | Memory capacity: | 120,000 data points / 120,000 sample values |
| Measurement point from the tip position: | 5 mm | Minimum sampling rate: | 1s |
| Precision: | Maximum sampling rate: | 24h | |
| -50 to -40°C: | ± 0.2 °C | Maximum startup delay: | 14 days |
| -40 to +140°C: | ± 0.1 °C | Benan: | Ex II1GD Ex ia IIC T3 Ga, -50 °C ≤ Tamb ≤ +105 °C |
| Sensor response time: | Time accuracy: | ±5s/24h | |
| T-63%: | 3.5 s | Battery: | TSP Standard Battery |
| T-90%: | 7.0 s | Please note: If the equipment is to be used in an ATEX environment, the special conditions for safe use specified in section 17 of the ATEX Certificate must be taken into account. | |
Sensor Options
4 Bar Pressure Sensor
6 Bar Pressure Sensor
Pressure and Rigid Temperature Sensor
Rigid Temperature Sensor
Bowie Dick Sensor
Double Rigid Temperature Sensor
Double Semi Flexible High Temperature Sensor
Double Semi Flexible Temperature Sensor
Internal Temperature Sensor
Rigid High Temperature Sensor
Rigid Multipoint Temperature Sensor
Rigid and SmartFlex Temperature Sensor
Rugged Flexible Temperature Sensor
Semi Flexible High Temperature Sensor
Semi Flexible Temperature Sensor
Vacuum Sensor
Automarker
Conductivity and Temperature Sensor
Double SmartFlex Temperature Sensor
High Range Conductivity and Temperature Sensor
Relative Humidity and Temperature Sensor
SmartFlex Temperature Sensor
Temperature Thermocouple Sensor
Key Benefits and Features
- One Logger - Multiple Sensor Options
- Conforms to 21 CFR Part 11
- Real-time data of SKY module
- User-friendly verification software
- Works with E-Val Pro thermocouple system
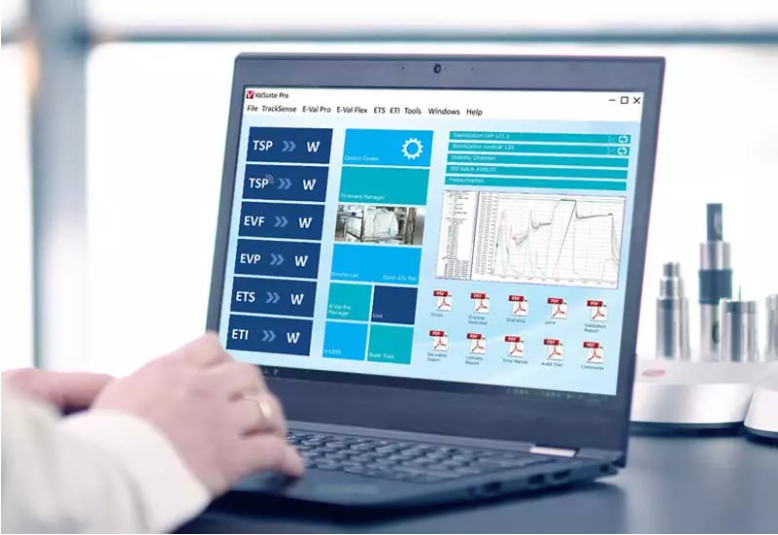
Software
ValSuite software is the industry's leading verification and calibration software and is highly credited with countless reporting options, impeccable data integrity, user calibration options and data analysis capabilities.
The software is used to run all of ELLAB's hardware and can combine various measurement systems into a unified study. In addition to its wide range of features, ValSuite complies with FDA 21 CFR Part 11 and is designed according to the GAMP guidelines. In addition, the software automatically generates PDF reports with clear pass/fail indications and can be run on stand-alone PCs or networked computers.
✓ Complies with FDA 21 CFR Part 11
✓ safe and reliable
✓ Windows Security Options
✓ Compatible with Windows 10
✓ Available in multiple languages
✓ Suitable for various applications
✓ Multiple Reporting Options
- One software platform for all Ellab devices
- Combine TrackSense data logger and E-Val Pro wired thermocouple system in the same session
- Automatic PDF report generation with clear pass/fail indication
- Runs on standalone PC or network
Applications

Environmental Test Chamber
Environmental test chambers are suitable for a variety of applications: product shelf-life, stability and packaging testing, light and temperature assessment studies, electronic component aging, plant growth, and more.
They are characterized by precise control of parameters such as temperature and relative humidity.

cold store
Refrigeration is used to prevent further biological activity, such as spoilage of food and medical products.The key parameter for the storage period is temperature, but often humidity is also monitored to understand drying conditions.

Hydrogen Peroxide
Hydrogen peroxide sterilization is a low-pressure, low-temperature, non-toxic process used in the pharmaceutical and medical industries to reduce the level of infectious agents. This process is preferred for products that cannot withstand the heat of a typical high-pressure sterilizer or have space for diffusion limitations, such as low-lumen medical equipment.
Key parameters include pressure/vacuum, transmitted RF energy, H2O2 concentration and temperature. Humidity can also be measured.

Steam sterilization
High-pressure sterilizers for the pharmaceutical and medical industries must meet established standards and specifications (EN 285 and ISO 17665) to ensure that their processes continue to provide safe and sterile results.
For this reason, autoclaves need to be identified and verified periodically to prove that they can sterilize within the qualified parameters.





