- Home
- Technical Products
Enterprise Cloud IT Solutions
Test Measurement
Industrial Measurement
- Solutions
Enterprise Cloud IT Solutions
Test Measurement
- Latest Articles
- About Us
 EN
EN
Chinese Pharmaceuticals Going Overseas
Globally Compliant Temperature Monitoring Solutions
Comply with FDA, EMA, WHO GDP/GMP certification requirements to ensure the quality and safety of pharmaceutical products in transnational transportation.
The rapid development of China's pharmaceutical industry has intensified competition at home, and companies need to explore overseas markets for growth. Policies support internationalization and encourage pharmaceutical exports and technology export. Global aging, increasing chronic diseases and growing demand for high-quality pharmaceuticals in emerging markets provide room for pharmaceuticals to go overseas. Chinese enterprises have enhanced their R&D, production and quality management capabilities, and are now competitive in the international arena. Integration of the global pharmaceutical industry chain and cross-country cooperation to promote the export of pharmaceutical products. Pharmaceuticals going overseas will help enterprises diversify risks, increase market share and brand influence; meet international standards (e.g. GMP, GxP) to enhance competitiveness; increase revenue from overseas high value-added demand; promote industrial upgrading; enhance international influence and voice; meet global patient demand and improve public health standards.
Industry Challenge: Temperature Monitoring Challenges for Pharmaceutical Exports
- Sensitivity of drugs to temperature and humidity
- Variability of the Transnational Transportation Environment
- Complexity of real-time monitoring and data management
- High standards of regulations and compliance in various countries.
API (API)
High temperatures may cause API degradation affecting efficacy. Degradation rate after 6 months of exposure at 40°C. KODAK 20%
Biological products (vaccines, antimicrobials)
Some vaccines are active after 1 hour of exposure to environments other than 2-8°C.Reduction of 10%-50%
Blood Products (Plasma, Stem Cells)
Over -20° C may lead to degradation of plasma proteins.Losing bets
Solving Pain Points
- Ensure the cross-border transportation and storage of pharmaceutical products comply with international regulations such as GMP and GDP, and reduce the risks of scrap, return and compliance caused by unstable temperature and humidity. Enhance the transparency and management efficiency of the supply chain through an intelligent and traceable monitoring system to ensure consistent standards, meet European and US GxP compliance requirements, and guarantee audit approval.
- In view of the sensitivity of drugs to temperature and humidity, a high-precision monitoring solution is adopted, which is integrated into the cold chain system to realize automated data collection and satisfy the requirements of FDA, EMA, etc. on the integrity of the data, so as to avoid compliance problems affecting the marketing of drugs.
- Comply with global cold chain standards to reduce the risk of transportation temperature control violations, respond quickly to anomalies through real-time monitoring and data tracking, optimize cost and service quality with efficient, integrable monitoring equipment, and reduce compliance risk by selecting suppliers with global certifications and success stories.

Avision ELPRO Solution: Full Process Temperature and Humidity Monitoring
Satisfy the strict requirements of temperature control in overseas markets, solve the difficulties in international transportation safety, help China's medicines to go overseas, empower the supply chain safety, and help provide patients with safe and effective medicines.
Industry Experience
0 +
Trusted by pharmaceutical companies worldwide
0 unyielding
GxP Compliance
0 %
Application Solutions
0 individual
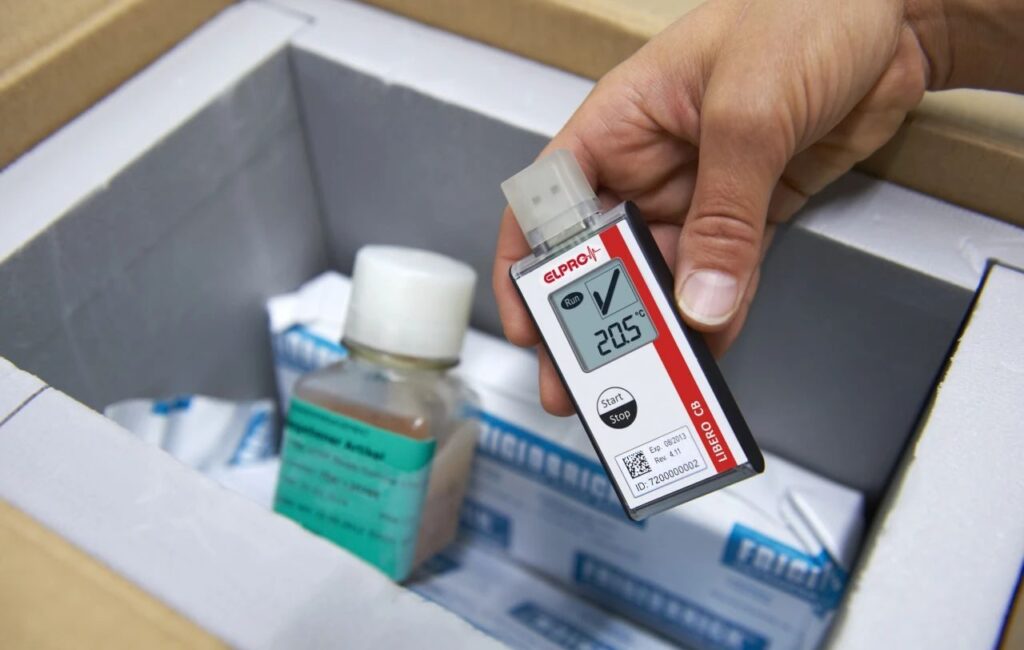
Your medication is safe with us
A globally compliant temperature and humidity monitoring solution is at the heart of ensuring drug quality and safety. With end-to-end temperature and humidity monitoring throughout the supply chain, HONGKE ELPRO can help you ensure quality and compliance at every step of the process, making it a reliable partner for your international drug export compliance!
Swiss quality, stable and reliable
More than 30 years of industry experience, trusted by the world's top 50 pharmaceutical companies, including Novo Nordisk, GSK, Bayer and Sanofi.
High price/performance ratio, easy to operate
Multiple settings to avoid mishandling, multiple combinations to suit all applications in the pharmaceutical supply chain
Global compliance, quality and safety
Comply with FDA, EMA, WHO GDP/GMP certification requirements to ensure the quality and safety of pharmaceutical products in transnational transportation.
Localized services to assist in data analysis
Customized services: support for configuration assistance, free trials, and compliance consulting services.
Product Recommendation
Export Non-Recycling Application
Export Recycling Multiple Applications

Multiple use Bluetooth transmission
PDF Temperature Recorder
Supports Bluetooth transmission and cell phone reading of PDF reports for fully automatic data management. Suitable for cold chain logistics, clinical trials, and temperature and humidity monitoring in a variety of temperature environments (ambient, refrigerated, frozen, dry ice, liquid nitrogen, etc.).
Export Real-time Transmission Application

Wireless Real-time Positioning
Temperature and Humidity Recorder
Embedded with IoT chip, real-time temperature and humidity tracking and location, safe air transportation. Ideal for cold chain transportation of high-risk goods such as high-value pharmaceuticals, cellular and gene therapy products, and global/clinical supply chain temperature and humidity monitoring.
Warehouse Laboratory Monitoring

GxP Compliant Automatic Ambient Temperature and Humidity Monitoring Systems
Suitable for warehouse, laboratory and clean room GxP environment monitoring, support multi-parameter monitoring, software to analyze, evaluate, archive and remote alarm, to help customers to comply with regulations to ensure product quality and patient safety.
Applicable Scenarios

Ingredients
(API)
Biological products
(Vaccines, Antibodies, Cell Therapy)
Blood Products
(plasma, serum, stem cells)

Preparations
(solid oral, injectable, freeze-dried preparations)

Clinical Trial Drugs
(IMP)
Compliance Standards and Certifications
- Coin cell battery powered, IATA compliant, recognized by more than 100 domestic and international airlines, added to the Air Cargo Clearance List.
- With the air and sea transportation authentication report of Shanghai Chemical Industry Institute and Beijing DGM, it can be directly put on the airplane.
- PDF/A proprietary technology, PDF data report is not modifiable and complies with FDA 21 CFR Part 11 requirements.
- WHO PQS pre-certified for direct temperature monitoring of vaccines during export transportation.
- Strictly based on GAMP5 R&D, design and production, in compliance with international and domestic pharmaceutical industry regulations.

Temperature deviation = drug failure? Intelligent Temperature Control Compliance Programs for Multinational Pharmaceutical Companies

Discover more solutions for temperature and humidity monitoring applications.
From multi-use and single-use USB temperature data loggers to real-time mobile Internet of Things and wireless Bluetooth (BLE) capabilities, Avision ELPRO offers solutions for any cold supply chain application in the pharmaceutical, life sciences, healthcare, and logistics sectors where disciplined temperature control is required to ensure quality results and compliance.
Testimonials
Reputation proves quality, trust comes from excellence.
"ELPRO provided us with the hardware and software solutions we were looking for to prepare for better data management. We were impressed with their ability to listen to our needs and then customize a solution for us. We appreciated the open dialog about the available solutions, our specialized needs, and ELPRO's future projects."

"Purchasing new equipment is often time-consuming and involves a lot of effort from selection to implementation. That's why we value a long-lasting, stable solution, and the ELPRO team has followed up with us to ensure that we have the right tools for the job, and ELPRO's solution provides us with a comprehensive approach to temperature monitoring for all of the processes that require high levels of compliance."



