- Home
- Technical Products
Enterprise Cloud IT Solutions
Test Measurement
Industrial Measurement
- Solutions
Enterprise Cloud IT Solutions
Test Measurement
- Latest Articles
- About Us
 EN
EN
TrackSense Pro - Relative Humidity and Temperature Sensor

Relative Humidity and Temperature Sensors
The relative humidity and temperature sensors have a temperature range of 0 to +90 °C and an accuracy of ± 0.1 °C (range of 10 to +90 °C).
The sensor has a relative humidity range of 0 to 100% RH with an accuracy of ± 2% RH (calibration range 10 to 90%).
It can be used for applications in the pharmaceutical industry and is particularly suitable for determining humidity levels in warehouses, stabilization chambers and during validation of EtO sterilization processes.
The high-quality wireless TrackSense Pro data logger is made of high-strength stainless steel and offers high-end technology for stable and highly accurate measurements in different thermal processes.
The TrackSense Pro DataCORDER series is equipped with an interchangeable sensor option that unlocks multiple measurement parameters:
✓ Temperature
✓ Pressure
✓ Conductivity
✓ Relative Humidity
✓ Vacuum
✓ Vapor penetration
The DataCORDER can be easily activated and read by multiple readout stations and combined with the Sky Module via wireless communication to obtain real-time data.
- Technical Parameters
| Sensors with this logger configuration: | Relative Humidity and Temperature Sensors | Recorders with this sensor configuration: | LAN 3G |
|---|---|---|---|
| RH measurement range (0 to +90 °C): | 0 - 100% (calibration 10-90%) | Working temperature: | 0 to +90°C |
| Measurement Principle: | Capacitive inductive/resistive | Operational pressure: | 0.001 mBar to 10 Bar ABS |
| Sensor element: | Capacitance/Pt1000 | Surface material: | 316 Liter Stainless Steel |
| RH accuracy at 25 °C without condensation (10% to 90%): | ± 2% | Diameter of the logger: | 25mm |
| RH Resolution: | 0.10% | Recorder length: | 44mm |
| RH Response Time (63% in slow moving air): | <15 s | With battery weight: | 48g |
| Temperature measurement range: | 0 to +90 °C (calibrated 10-90 °C) | Memory capacity: | 120,000 data points/60,000 sample values |
| Temperature accuracy: | Minimum sampling rate: | 1s | |
| +10 to +90°C: | ± 0.1 °C | Maximum sampling rate: | 24h |
| Temperature resolution: | 0.01°C | Maximum startup delay: | 14 days |
| Temperature response time: | Benan: | Ex II1GD Ex ia IIC T3 Ga, -50 °C ≤ Tamb ≤ +105 °C | |
| T-63%: | 6.5 s | Time accuracy: | ±5s/24h |
| T-90%: | 17 s | Battery: | TSP Standard Battery |
| *Requires ValSuite 5.2 or later. | Please note: If the equipment is to be used in an ATEX environment, the special conditions for safe use specified in section 17 of the ATEX Certificate must be taken into account. | ||
Sensor Options
4 Bar Pressure Sensor
6 Bar Pressure Sensor
Pressure and Rigid Temperature Sensor
Rigid Temperature Sensor
Bowie Dick Sensor
Double Rigid Temperature Sensor
Double Semi Flexible High Temperature Sensor
Double Semi Flexible Temperature Sensor
Internal Temperature Sensor
Rigid High Temperature Sensor
Rigid Multipoint Temperature Sensor
Rigid and SmartFlex Temperature Sensor
Rugged Flexible Temperature Sensor
Semi Flexible High Temperature Sensor
Semi Flexible Temperature Sensor
Vacuum Sensor
Automarker
Conductivity and Temperature Sensor
Double SmartFlex Temperature Sensor
High Range Conductivity and Temperature Sensor
Relative Humidity and Temperature Sensor
SmartFlex Temperature Sensor
Temperature Thermocouple Sensor
Key Benefits and Features
- One Logger - Multiple Sensor Options
- Conforms to 21 CFR Part 11
- Real-time data of SKY module
- User-friendly verification software
- Works with E-Val Pro thermocouple system
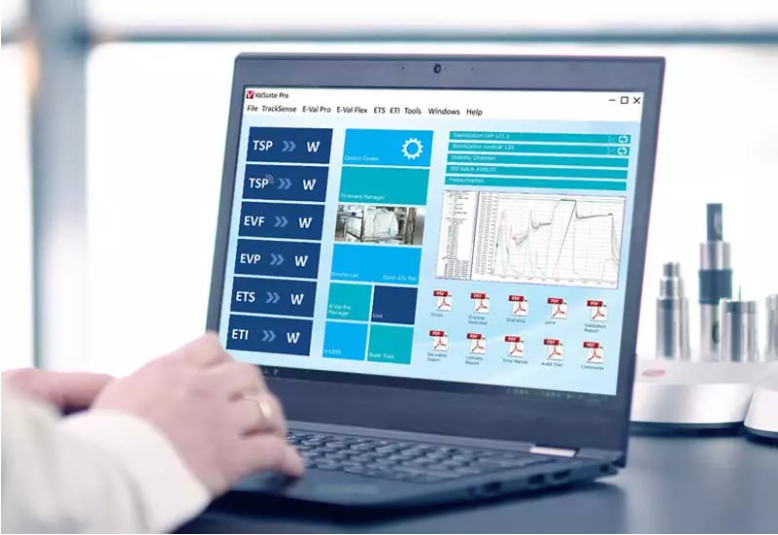
Software
ValSuite software is the industry's leading verification and calibration software and is highly credited with countless reporting options, impeccable data integrity, user calibration options and data analysis capabilities.
The software is used to run all of ELLAB's hardware and can combine various measurement systems into a unified study. In addition to its wide range of features, ValSuite complies with FDA 21 CFR Part 11 and is designed according to the GAMP guidelines. In addition, the software automatically generates PDF reports with clear pass/fail indications and can be run on stand-alone PCs or networked computers.
✓ Complies with FDA 21 CFR Part 11
✓ safe and reliable
✓ Windows Security Options
✓ Compatible with Windows 10
✓ Available in multiple languages
✓ Suitable for various applications
✓ Multiple Reporting Options
- One software platform for all Ellab devices
- Combine TrackSense data logger and E-Val Pro wired thermocouple system in the same session
- Automatic PDF report generation with clear pass/fail indication
- Runs on standalone PC or network
Applications

Environmental Test Chamber
Environmental test chambers are suitable for a variety of applications: product shelf-life, stability and packaging testing, light and temperature assessment studies, electronic component aging, plant growth, and more.
They are characterized by precise control of parameters such as temperature and relative humidity.

Ethylene oxide sterilization
Ethylene oxide sterilization is a low temperature process used in the pharmaceutical and medical industries to reduce the level of infectious agents.This process is preferred for products that cannot withstand the high temperatures of typical autoclaves.Key parameters include temperature, humidity and pressure levels.

Hydrogen Peroxide
Hydrogen peroxide sterilization is a low-pressure, low-temperature, non-toxic process used in the pharmaceutical and medical industries to reduce the level of infectious agents. This process is preferred for products that cannot withstand the heat of a typical high-pressure sterilizer or have space for diffusion limitations, such as low-lumen medical equipment.
Key parameters include pressure/vacuum, transmitted RF energy, H2O2 concentration and temperature. Humidity can also be measured.

Warehouse
Warehouse environments in the pharmaceutical, medical and food industries may require regulatory compliance, validation and monitoring.
Temperature and humidity are the most common critical parameters that cannot exceed certain limits and need to be recorded accurately.





