
【虹科動態】 虹科×長城汽車技術交流日回顧 – 智慧汽車、車載以太網、GNSS 測試全鏈路方案
2025年虹科攜手長城汽車於保定技術中心舉辦技術交流日,分享智慧汽車、車載以太網、GNSS測試、T-Box通信及aiSim仿真全鏈路方案,展示前沿技術應用與創新成果,助力汽車通信與智慧駕駛行業發展。
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
With the rapid development of the medical device industry and increasingly stringent regulatory requirements, how to ensure that the production and storage environments fully comply with industry standards has become an important issue for enterprises. High-precision temperature and humidity monitoring is not only the key to ensure product quality, but also the core requirement for enterprises to realize compliance management and risk control.
Medpac Medical Device Manufacturing (Changzhou) Co.elproMONITOR Wired Automatic Temperature and Humidity Monitoring SystemThe elproMONITOR monitoring system is designed to meet the complex and demanding environmental control needs of medical device manufacturers. In this article, we will introduce how Hongke elproMONITOR monitoring system can help medical device manufacturers to establish an efficient and reliable environmental monitoring mechanism to ensure product quality and regulatory compliance, and promote enterprises to move steadily towards intelligent manufacturing and digital transformation.

Ypsomed is a leading developer and manufacturer of self-injection and delivery systems with a strong reputation in the field of diabetes solutions. Ypsomed's customers include a number of internationally recognized pharmaceutical companies, including AstraZeneca, Bristol-Myers Squibb, Pfizer, Roche, Genentech and Toho.
Located in the Life and Health Industrial Park of Changzhou Hi-Tech Zone, Medpac Medical Device Manufacturing (Changzhou) Co., Ltd. is Medpac's first production base in the Asia-Pacific region, with a total investment of more than EUR 40 million, specializing in the research, development, production and assembly of medical syringe pens and needles. The plant is expected to commence production in 2024, with an annual production capacity of 20 million UnoPen portable self-administration injection devices, mainly for insulin and other multi-dose therapeutic regimens, and will be delivered to well-known global pharmaceutical companies for pre-filling.
In the future, the Changzhou plant will adhere to the concept of sustainable development, introduce a fully automated mass production system, and build a high-specification smart factory that meets the standards of Industry 4.0.

Driven by the trend of "Smart Manufacturing" and "Industry 4.0", Medpac Medical Device Manufacturing (Changzhou) Co., Ltd. puts forward higher level requirements for production environment control and data monitoring, and its environmental monitoring system must meet the following core requirements:
The production of medical devices must comply with GMP (Good Manufacturing Practice), FDA 21 CFR Part 11, and other international regulations to ensure that the temperature and humidity in the clean area and assembly workshop are stable over time.
The system should be equipped with automatic monitoring and real-time warning function, when the temperature and humidity exceed the preset range can immediately send out an alarm to ensure that the relevant personnel can quickly take countermeasures to avoid environmental fluctuations affect the quality of products.
The environmental monitoring system must comply with FDA 21 CFR Part 11 to ensure the authenticity, integrity and traceability of the monitoring data. The data must be protected from tampering and audit trails, and support real-time collection and secure storage.
Comply with international standards such as GMP, GLP, GAMP5, FDA 21 CFR Part 11, Annex 11 and HACCP.
Supports fully automated data monitoring, control, report generation, data archiving and alarm management.
Early identification of potential risks to avoid product and asset losses
Adoption of high-precision and high-stability measurement equipment and software system.
Swiss-engineered, low total cost of ownership for R&D, production and storage facilities
Provide professional technical support and maintenance services, which can be customized according to the actual needs.
Convenient system setup and remote access support for all types of auditing needs.
Powerful user management, real-time notifications and automatic report generation for facilities of all sizes
Complete recording of key environmental parameters for data-driven and paperless management.
Providing GxP Consultation and Validation Services
Professional and compliant project and calibration services
Long-term reliable partner of the world's top 50 pharmaceutical companies
The Grant DEN-1 and DEN-1B turbidimeters have become indispensable tools for pharmaceutical microbiological testing, clinical diagnosis, food safety monitoring, and basic research with their accurate turbidity measurement capability, fast detection speed, intuitive and user-friendly interface, flexible sample management, and highly competitive price/performance ratio of imported brands. Combined with the professional and immediate technical support services provided by PointChem, Grant Turbidimeters provide a reliable solution for laboratories in pursuit of high efficiency, accuracy, and regulatory compliance in quality control.
It can be integrated into an organization's existing IT infrastructure and database to support centralized monitoring of hundreds or even thousands of measurement points. elproMONITOR software manages measurement data, alarms, and reports with a user-friendly, intuitive interface, and provides compliance verification to help users complete their daily operations quickly and efficiently.
ECOLOG-PRO LBR Communication and Power Modules: Supports RJ45 interface for centralized powering of up to 32 measurement modules.
ECOLOG-PRO 4PT Temperature Measurement ModuleConnects 1-4 Pt100 probes with measurement range of -200 to +200°C and accuracy of ±0.2°C.
ECOLOG-PRO 2TH Temperature and Humidity Measurement Module: 1-2 temperature and humidity probes can be connected.
ECOLOG-PRO 4MA Analog Measurement Module: Suitable for monitoring environmental parameters such as CO₂, differential pressure, etc.
ECOLOG-PRO 4DO Digital Output ModuleProvides local audio and visual alarm outputs, supports SMS and e-mail alarms.
ECOLOG-PRO 4DI Digital Input Module: For access control status monitoring with data storage function.
"We are very satisfied with the overall performance of the Elpro wired monitoring system in our plant. The system was easy to install, simple to operate and fully functional, especially in terms of real-time temperature and humidity monitoring and data logging, providing us with a comprehensive overview of our production environment. The alarm mechanism of the system responds instantly, helping us to identify and deal with potential environmental risks in the first instance, and ensuring the quality and safety of each batch of medical device products.".
- Dr. Pui Sang

2025年虹科攜手長城汽車於保定技術中心舉辦技術交流日,分享智慧汽車、車載以太網、GNSS測試、T-Box通信及aiSim仿真全鏈路方案,展示前沿技術應用與創新成果,助力汽車通信與智慧駕駛行業發展。

虹科 GNSS 模擬器提供消費電子、導航晶片與定位終端測試解決方案,精準模擬全球衛星導航信號(GPS、GLONASS、Galileo、北斗),提升定位精度、時間同步與數據處理效率,保障產品質量並縮短研發周期。
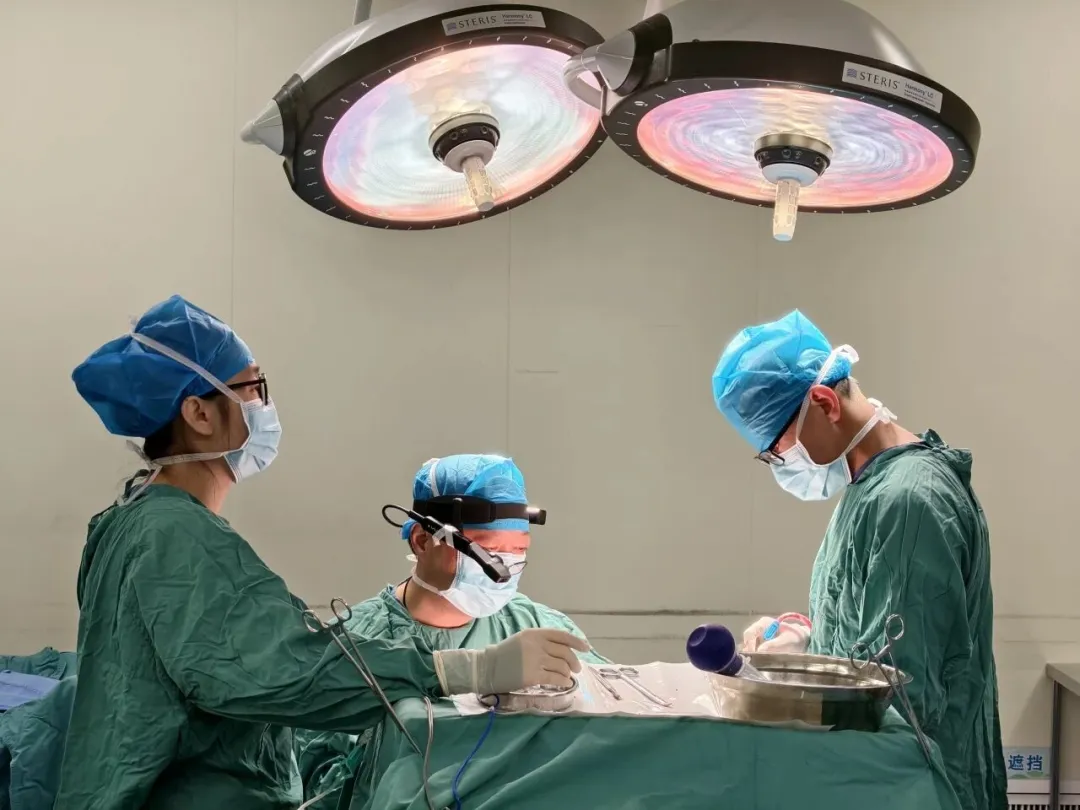
虹科基於醫療級 AR 智慧眼鏡的遠端醫療解決方案,成功實現中國首例 AR 遠端顱骨修復手術指導。憑藉 ISO 無塵室與 EMI 雙重醫療認證,協助推動分級診療,全面提升手術安全性與醫療協作效率。